Can aging be cured? Eternal youth has long been a dream. Now, through modern science, humanity is on the verge of curing aging and achieving immortality.
The pyramids were built to grant eternal life to the pharaohs buried within them. China’s first emperor, Qin Shi Huang, sent 1,000 people out on ships to find the elixir of life — none returned.
These examples show how far people will go to escape their fate.
Our oldest recorded story, the Epic of Gilgamesh, is 4,100 years old. It details one king’s quest for immortality. Failing in that, he finds a plant that promises rejuvenation, only for it to be stolen by a serpent.
The 3,500 year old Rigveda, of the Hindu tradition, describes a drink of the Gods called amrita. It confers to its drinkers knowledge and immortality. The demon snake Rahu tried but failed to steal it.
In the Book of Genesis, compiled 2,600 years ago, a serpent tricked Adam and Eve into eating from the tree of knowledge. For this God banished humans from paradise. Humans thereby lost access to eat from the tree of life, which conferred immortality.
Even science tells a story of immortality lost.
Early in our evolutionary lineage we were single-celled organisms. These cells did not age. They were immortal. But with the advent of sexual reproduction, individual immortality was no longer necessary for the continuation of the species. And so immortality was lost.
People have long dreamed of returning to this immortal state. To eat the food of the gods and to bathe in the fountain of youth.
But is this dream attainable?
Eternal youth through supernatural means might forever be a dream, but what about eternal youth through the magic of technology?
Religion talks about transcending death, but it has a mystical answer to how that happens. In fact, we find this transcendence in the real physical world. We find it in technology. If you put materials and energy in the right configurations, magical things happen.
Ray Kurzweil
Are there medicines or treatments that could reverse aging and restore youth? Is eternal life technically possible?
If such therapies can be developed and made available in your lifetime, then you can consider yourself already immortal. For with these therapies there is no limit to how long you might inhabit this world.
We are closer to realizing these treatments than is generally realized. Age-reversing drugs have already seen human testing.
Contents
A maximum lifespan?
How come no one lives to celebrate their 200th birthday?
Even if a person avoids serious injury and disease, their body still gives out around a maximum of 120 years.
It’s like we are built with planned obsolescence — like a smartphone that gives out the day after it’s warranty expires. No matter how careful one is or how well one takes care of their body, death comes for all.
But there are reasons to be hopeful. For one, there is no biological, chemical, or physical law that requires lifeforms to eventually die.
There is nothing in biology yet found that indicates the inevitability of death. This suggests to me that it is not at all inevitable, and that it is only a matter of time before the biologists discover what it is that is causing us the trouble and that that terrible universal disease or temporariness of the human’s body will be cured.
Richard Feynman, “The Pleasure of Finding Things Out” (1999)
The disease of aging
There is a disease that affects 100% of the population. Worse, it’s universally fatal: the disease of aging.
Aging is not usually considered a disease. But it is a disease in the literal sense of the word. Ageing certainly causes dis-ease.

Ageing impairs the immune system, increases healing time, reduces muscle mass, dulls the senses, and lowers energy.
I grabbed a pile of dust, and holding it up, foolishly asked for as many birthdays as the grains of dust, I forgot to ask that they be years of youth.
Ovid, in Metamorphoses (8 A.D.)
We may not be able to stop the accumulation of years as time marches on. But perhaps, it is not necessary that our bodies become increasingly frail with the passage of time.
The body as a machine
The fields of medicine and anatomy regard the human body as a machine — a sophisticated one, but a machine nonetheless.
This view took shape in the 1600s:
I should like you to consider that these functions (including passion, memory, and imagination) follow from the mere arrangement of the machine’s organs every bit as naturally as the movements of a clock or other automaton follow from the arrangement of its counter-weights and wheels.
René Descartes, in Treatise on Man (1633)
For what is the Heart, but a Spring; and the Nerves, but so many Strings; and the Joynts, but so many Wheeles, giving motion to the whole Body, such as was intended by the Artificer?
Thomas Hobbes, in The Leviathan (1651)
Each body part has its own function. The heart a pump, the nerves wires, the fat a fuel tank, the lungs an air intake, kidneys an exhaust system, muscles motors, the brain a computer.
Aging as wear and tear
A major accident is not required for a car to break down and stop working. A car can break down from lack of maintenance and repair.
The car dies from the accumulation of many small injuries, which we call wear and tear. The balding of tire treads, the build up of particles in motor oil, the grinding of gears, clogging of filters — they all add up.

Individually, none of these is fatal. But it leads to death by a thousand cuts. It is the accumulation of many small injuries we don’t see.
Aging is similar.
As cellular machines, our bodies have two parts: (1) cells and (2) the stuff between cells. Accordingly, any persistent damage to our machinery manifests either in the cells or in the stuff between them.
Between 1955 and 1982 science went from having almost no understanding of the mechanisms of aging to having what is now considered a complete picture. No new forms of age-related damage have been discovered since 1982.
Age-related damage falls into seven categories. The aging researcher and biomedical gerontologist Aubrey de Grey has nicknamed these the seven deadly sins of aging:
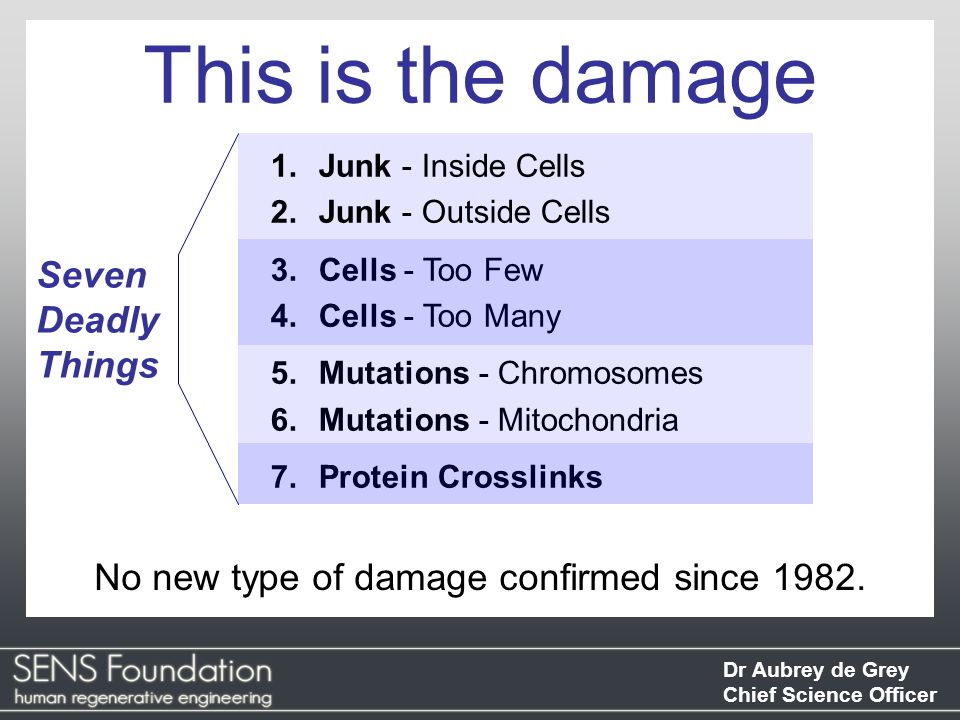
Seven types of damage are responsible for all the effects of aging:
- Junk inside cells: Every cell in your body has its own “stomach” called a lysosome. Lysosomes break down harmful and useless molecules in the cell. But not every molecule can be perfectly digested. This lysosomal waste builds up as lipofuscin granules. Like dirt in motor oil, this gunks up the machinery of the cell, causing diseases like macular degeneration and Parkinson’s disease.
- Junk outside cells: Junk can accumulate in the spaces between cells. Examples include the build up of atheromatous plaques in arterial walls that cause atherosclerosis and associated cardiovascular diseases. Similarly, neuritic plaques accumulate around brain cells and are thought to play a role in Alzheimer’s disease.
- Too few cells: After dividing about 50 times, human cells become senescent and stop dividing. This leads to many dysfunctions. The heart weakens as heart cells aren’t replaced fast enough. Organs like the kidney and liver become less efficient as cells die and are not replaced. Loss of pancreatic beta cells leads to diabetes. Loss of pigment producing cells causes us to gray.
- Too many cells: Senescent cells often become dysfunctional. They not only sit around doing nothing, they become dead weight. They use resources that could be used by healthy and functional cells. These loiterers cause sub-optimal function of our tissues and organs. They can also secrete harmful proteins that cause cancer in surrounding cells.
- Mutations in chromosomal DNA: Mutations to our nuclear DNA can cause cellular dysfunction. The most severe form occurs when tumor suppressor genes are damaged. This leads to tumors and cancer — both of which result from uncontrolled cell division.
- Mutations in mitochondrial DNA: Mitochondria are the power plants of our cells. They produce the energy molecule ATP and are responsible for body heat. Mutations in mitochondrial DNA impact a cell’s ability to function and reduce metabolic efficiency. The membranes of mitochondria become leaky, and like having a hole in a fuel line, less gas makes it to the engine.
- Protein crosslinks: Protein crosslinks are the scaffolding that hold our cells together. Without them, you would fall apart into a pile of goo. These crosslinks are made of proteins like collagen and elastin. Over time, sugar molecules bind to these proteins causing them to stiffen. Loss of elasticity is responsible for wrinkles, hardening of arteries, while hardening of the lens causes farsightedness.
Is it possible to undo all these forms of age-related damage?
Immortality in Nature
If immortality and eternal youth are possible, why don’t we see them?
Surely some species would have figured it out. But then why don’t we find million-year-old trees and thousand-year-old animals?
Why aren’t there creatures that maintain a youthful vigor throughout their lives? Surely that would convey some evolutionary advantage.

Actually, biologists have found species that don’t weaken with age. These species only seem to get bigger and stronger with time.
Forever young
Some species experience negligible senescence — they show little to no diminishment with age.
These animals include sea anemones, ocean quahogs, lobsters, rougheye rockfish, Greenland sharks, and naked mole rats.

Image Credit: Alma Lobster Shop
Besides showing no signs of aging, these creatures are long-lived.
Naked mole rats, despite being the size of mice, live 8 times longer. Unlike all other mammals, the annual chance of dying for a naked mole rat does not increase as they age — it stays constant.
The oldest known ocean quahog was 507 years old and the oldest Greenland shark was estimated to be as old as 512 years.
Biological immortality
Negligible senescence shows that some organisms can avoid or repair the damage that comes from aging. Despite this, these creatures still eventually die. They are long-lived but they are not immortal.
Is it possible for organisms to not only remain young, but live forever?
Immortal organisms
A truly immortal creature would not just live for hundreds of years, but millions or billions of years.
In 1995, the microbiologist Raul Cano revived yeast that had been trapped in amber for 45 million years.
Image Credit: Wikimedia
These yeast cells survived over geological periods of time. In 2011, Cano achieved his dream and used these yeast to craft beer.
Scientists have sprouted 31,800 year old seeds and revived worms that laid frozen in Siberia for 42,000 years. Bacteria spores, trapped in a salt crystal for a quarter billion years, have been returned to life.
As remarkable as these cases are, none of these organisms were fully alive during these times. They were not eating, breathing, or reproducing. They idled in a dormant state, waiting for reactivation.
The immortal life forms we seek aren’t in some kind of stasis, but should be actually living for millions of years. They need to be metabolically active — eating, digesting, breathing, moving, reproducing.
As it happens, there are creatures on this planet that have lived billions of years. Some can probably be found in locations not far from you.
Immortal Cells
This amoeba is between 750 million and 1.5 billion years old.
This very same amoeba predates all fish and plants. It is older than the continents. It watched the dinosaurs rise and fall.
We know it is ancient because some of its cousins left behind fossils.
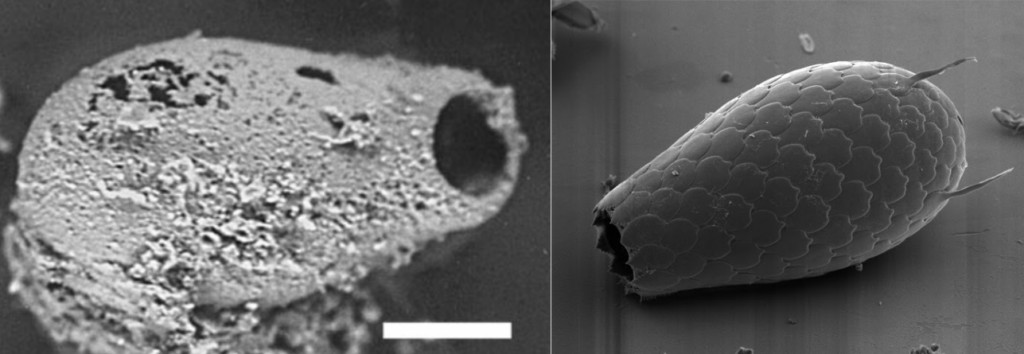
How could this creature have survived for so long? How could it always be in the right place and time to avoid being stepped on or eaten?
It all comes down to how amoebas reproduce.
Amoebas reproduce by splitting in half. So then, which of the two is the original? Both are made entirely from atoms and molecules of the original. Both have equal vitality. Neither is mother or daughter.
Accordingly, you can start from any amoeba alive today and trace its history back through the eons. Eventually this backwards trace will reach something too unlike an amoeba to still be called one — perhaps at a time around 1.5 billion years ago when their kind, known as protists, were the dominant life on earth.
Every amoeba alive today is the first amoeba. All are equally ancient.
That First Amoeba, weirdly clever,
Arthur Guiterman in “Ode To The Amoeba” (1922)
Exists today and shall forever,
Because he reproduced by fission;
He split himself, and each division
And subdivision deemed it fitting
To keep on splitting, splitting, splitting;
So, whatsoe’er their billions be,
All, all amoebas still are he.
Humans often consider themselves the pinnacle of evolution. But the amoeba’s genome is hundreds of times larger than ours.
The amoeba has had much time to learn tricks to survive in the varying conditions Earth has produced over the past billion years.
One of those tricks is the secret to immortality and eternal youth.
We share a common ancestor with the amoeba. Amoeba are our cousins. Perhaps we have retained in ourselves some of the genes necessary to live forever. Genes to clear cellular junk, and rejuvenate old cells. Perhaps there is a way to reactivate these ancient genes.
But the amoeba, unlike our bodies, is just a single cell. It doesn’t have to deal with junk building up between cells. Is there any evidence of immortality in multicellular life?
Are there immortal plants and animals?
Immortal Animals
The Hydra of Lerna is a creature of Greek and Roman mythology. It protects the entrance to the underworld.
The Hydra has many snake-like heads, it lives in the water and can regenerate: if any head is cut off, two more grow in its place.

The Hydra of Lerna was a myth, but there is a real-life hydra.
It was first described by the “Father of Microbiology” Antonie van Leeuwenhoek in 1702. Like the hydra from mythology, this hydra is aquatic and has many snake-like appendages.
In 1744, Abraham Trembley discovered the hydra’s regeneration abilities. If a hydra’s arm is cut off, not only will it grow back, but the severed limb will regrow into a second hydra.
In 1971, researchers took this to the extreme. They found that hydra could be cut into hundreds of pieces, and found every clump with at least a few hundred cells reassembled into a new hydra.
In 1998, a study concluded that hydra may even be immortal.
The results provide no evidence for aging in hydra: mortality rates have remained extremely low and there are no apparent signs of decline in reproductive rates. Hydra may have indeed escaped senescence and may be potentially immortal.
Biologist Daniel E. Martínez in his study of hydra (1998)
Like the amoeba, hydra reproduce by splitting. But the vitality of the budded-off individual is no more or less than the original. This suggests that hydra, like amoeba, are immortal.
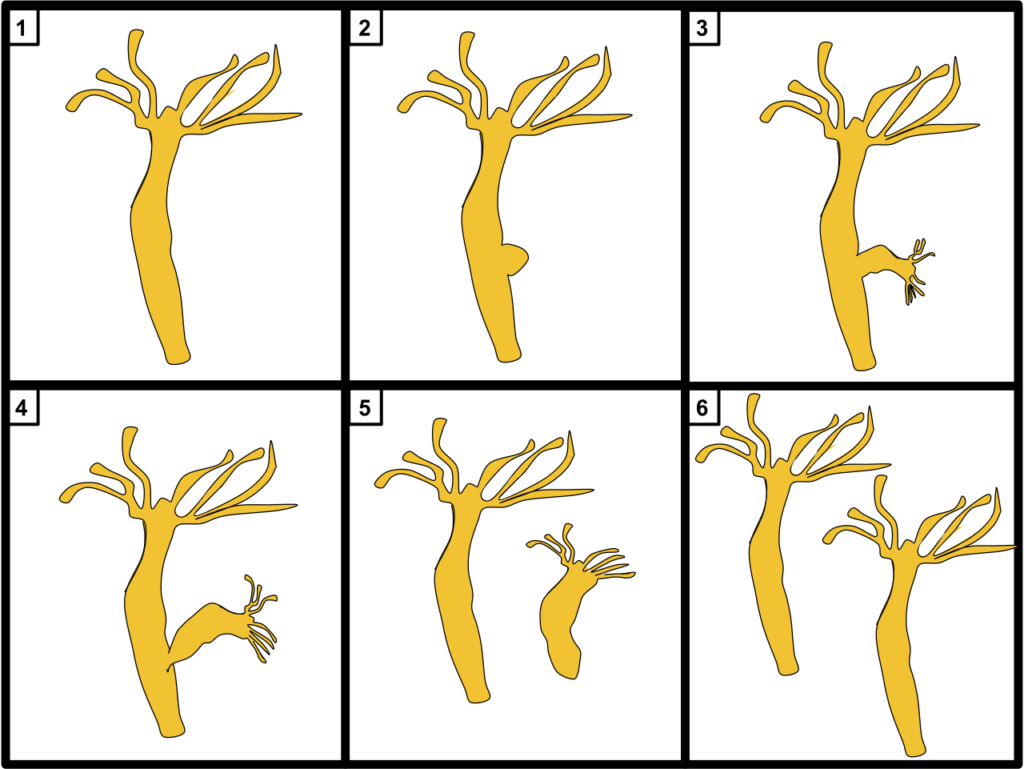
But unlike amoeba, hydra are animals.
While small, they contain tens of thousands of cells and can grow to several centimeters — large enough to be seen by the naked eye.
Their closest relatives are sea anemone and jellyfish.
Hydras owe their regeneration ability to the fact that they are filled with stem cells. Some even describe hydra as nothing but stem cells.
But hydra are tiny. Can immortality exist in large lifeforms?
Immortal Plants
The largest known living thing is not a whale, nor a sequoia tree, but something much bigger. It takes up 100 acres and weighs 6,600 tons — 1,000× more than an elephant and 3× more than the largest sequoia.
In addition to being the largest organism, it is also one of the oldest. It’s 80,000 years old. Like the hydra it is believed to be immortal.
This organism is a plant. It is a vast interconnected root structure, which manifests above ground as an aspen forest.

What we perceive to be individual aspen trees are more like branches of the same tree. These “branches” spring up from underground, and can appear up to 100 feet away as root suckers spread through the soil.
The largest known aspen tree system is called Pando. Found in Utah. Genetic testing revealed all trunks belong to the same organism.

The immortality of hydra and aspens tells us immortality doesn’t require being single-celled, small, or a plant.
Immortality only requires mechanisms for renewal.
Immortal species
When we stop and think, we realize that all species are immortal.
Every species has mechanisms for rejuvenation. Some rejuvenate themselves perpetually, like the amoeba, hydra, and aspens.
Others, like the immortal jellyfish, rejuvenate themselves periodically, reverting from adulthood back to an immature state.
Still other species rejuvenate during sexual reproduction, where two adult cells from the parents come together to form a new cell that is reset to a younger state.
Somehow this process “resets the clock” on the cell and enables them to continue dividing for another human lifetime.
In this way every human cell belongs to a long, unbroken chain of human cellular divisions. This chain goes back millions of years.
While no human individual is immortal, humanity as a whole, is.
This is an important clue. Within us are mechanisms to reset human cells to a younger non-senescent state. These rejuvenated cells figure out how to clear cellular junk, restore DNA, and resume dividing.
If we could figure out the mechanisms for triggering this cellular reset, it might be possible to trigger those mechanisms in our existing cells.
In 2006, the researcher Shinya Yamanaka discovered how to do this. He identified four proteins, known as transcription factors, which when applied to a cell can reset it to a younger state.
Using these four transcription factors — now called Yamanaka factors, He could program any cell to revert back to a pluripotent stem cell.
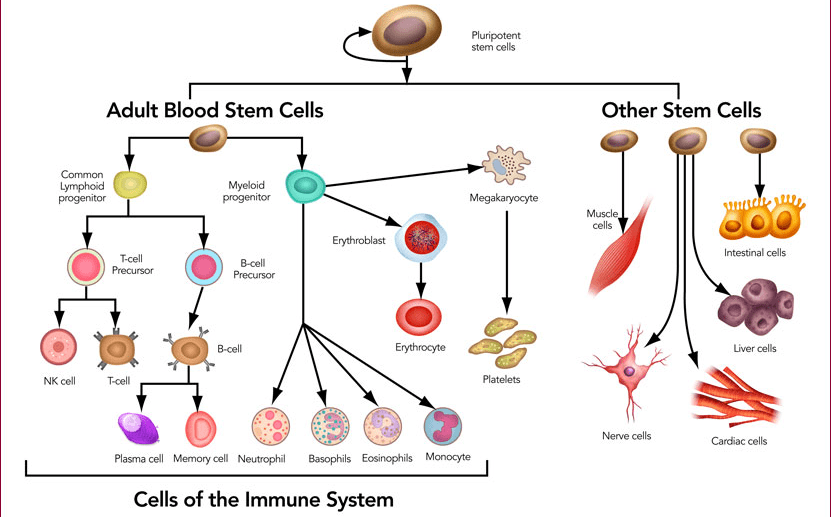
These are cells in a state before they differentiate into separate types, such as skin cells, muscle cells, liver cells, brain cells, etc.
Stem cells are the cells that existed in the human embryo before they differentiated into our separate tissues and organs.
In 2012, Yamanaka received the Nobel prize in Medicine “for the discovery that mature cells can be reprogrammed to become pluripotent.”
Stem cells are the basis of the emerging field of regenerative medicine.
Maintaining the machine
How long can a car be kept on the road?
With proper care, and maintenance there is no upper bound for how long a car can be kept in working order.
Any damaged part that can’t be repaired can be replaced.
Replacing the parts
But what if parts couldn’t be replaced?
Imagine a great shortage of replacement parts for automobiles. A lack of spare fuel pumps, carburetors, tires, headlights, etc.
Many otherwise functioning and serviceable cars would end up in the scrap heap, all because it had a bad part that couldn’t be replaced.
This is the situation for our bodies. The lack of spare parts is now the leading cause of death in developed nations. In the United States, 35% of deaths — nearly a million people a year — are due to the unavailability of organs. The problem has been getting worse.
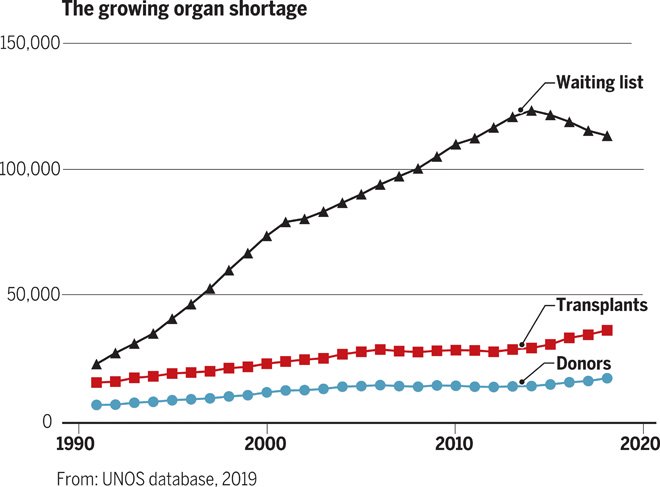
Of the 30 million people diagnosed with heart disease in the U.S., fewer than 1 in 1,000 ever receives a heart transplant.
But thanks to recent discoveries and technologies there is hope. All these deaths become preventable when we can create new organs on demand through regenerative medicine.
Virtually any disease that results from malfunctioning, damaged, or failing tissues may be potentially cured through regenerative medicine therapies.
U.S. Department of Health and Human Services in “2020: A New Vision – A Future for Regenerative Medicine” (2005)
On-demand organs
The existing approach to organ transplant is less than ideal.
Only 1 person in 300 dies in a way that allows organ donation. Moreover, for donation to succeed requires a close match. This has led to long waiting lists.
Further, there is the chance of organ rejection, where the immune system attacks the organ as a foreign body. To reduce the chance of this, the recipient must be put on immunosuppressive drugs for life.
New technologies allow for better ways. Replacement organs can be grown from the patient’s own cells, not only guaranteeing availability but also a perfect match — it eliminates any chance of rejection.

The technique of organ printing combines two base technologies:
- Reprogramming adult cells to stem cells and then to other cell types
- 3D printers that fabricate arbitrary 3-dimensional shapes with bioink
It starts by converting a patient’s own cells into stem cells. These stem cells are then reprogrammed into cells of any kind. Different cell types are used as ‘bioink’ in a 3D printer that prints them into any shape.
Some organs are comparatively simple. A bladder is composed of just 2 cell types, but a kidney has over 30. Nevertheless, complex organs have been successfully printed and even implanted.
In 2015, Organovo bioprinted human livers for use in drug testing by Merck. In 2018, the axial3D helped doctors in Ireland fashion the first bioprinted kidney to be implanted in a human patient.
Organ printing is a game changer. It will eliminate the need for immunosuppressive drugs, waiting lists, and organ rejection. People will no longer have to die from a lack of spare parts.
You die because your organs break. If we can start replacing them, we can extend life.
Erik Gatenholm, CEO the bioprinting company Cellink
Regenerative medicine technologies like bioprinting will extend and improve the lives of many. But as incredible as bioprinting is, it’s only the beginning for what will soon be possible.
Repairing the damage
Replacing organs requires surgery, which is expensive and risky.
A less invasive form of maintenance is to repair organs and tissues in place. To cause them to heal and rejuvenate themselves.
It is akin to getting a tune-up rather than replacing the engine.
Gene therapies and drugs may soon allow us to get tune-ups for our bodies. This can restore us to a healthier more youthful state.
Gene therapies for repair
Genes are the programming that govern the activities of every one of our cells. Gene therapies seek to modify the genes or how they are expressed, by adding, activating, deactivating, or deleting genes.
Tests of gene therapies on the lifespans of other species have produced incredible results. Below is a sample of what has been achieved so far.
Yeast
In 2008, researchers knocked out two genes, RAS2 and SCH9, found to promote aging and cancer in humans. They applied this gene modification to a population of yeast.
We got a 10-fold life span extension that is, I think, the longest one that has ever been achieved in any organism.
USC biologists and leader of the study Valter Longo
Worms
In 1993, biologists knocked out a single gene called DAF-2. The gene controls insulin-like growth factor receptors and is present in humans.
Disabling this one gene doubled the lifespan of the worms.
In 2013, scientists went further. In addition to inhibiting DAF-2, they blocked RSKS-1, which controls nutrient signaling pathways. They expected to get a 130% increase in lifespan, but were shocked when they found the worms lived five times longer.
The two mutations set off a positive feedback loop in specific tissues that amplified lifespan. Basically, these worms lived to the human equivalent of 400 to 500 years.
Dr. Pankaj Kapahi who led the study
Mice
In 2008, scientists genetically modified mice to produce more of a DNA protecting enzyme called telomerase. They lived 50% longer, and moreover, stayed younger and healthier longer.
In 1998, a dwarf mouse was genetically modified to have its Growth Hormone Receptor (GHR) gene knocked out (KO). It was given the designation GHR-KO 11C.
As a result of this treatment the mouse was smaller than average, and also showed reduced insulin and blood sugar.
But this mouse lived one month shy of its 5th birthday. This is more than double the normal lifespan for these mice (2.25 years).
Mice like GHR-KO “Dwarf” Mouse 11C aren’t born every day, but the lessons they teach to science are invaluable.
Andrzej Bartke, who worked with the mouse
Drugs for repair
Gene therapies remain highly experimental and risky. They are much harder to reverse. As a result, pharmaceutical interventions for life extension are farther along when it comes to human testing.
Eliminating senescent cells
In 2016, researchers from the Mayo Clinic found that systematically removing senescent cells with twice-weekly injections of AP20187 extended the lives of mice by 25 percent.
It also delayed the onset of age-related illnesses in the mice.
It’s not just that we’re making these mice live longer; they actually stay healthier longer too. That’s important, because if you were going to equate this to people, well, you don’t want to just extend the years of life that people are miserable or hospitalized.
Author of the study Darren Baker, in Popular Mechanics interview
After the study came out, Nathaniel David founded the Unity Biotechnology develop similar treatments for humans.
If we can translate this biology into medicines, our children might grow up in a different world than we did.
Nathaniel David, CEO of Unity Biotechnology in Fortune Magazine
Repairing DNA
The chemical NAD+ can be found in every cell in your body, but it declines with age. It is believed to play a role in protecting DNA against damage.
In 2017, an international team led by David Sinclair found that after a single week of treatment with a chemical called NMN–which converts to NAD+ in the body–the cells of older mice were restored to youth.
The cells of the old mice were indistinguishable from the young mice, after just one week of treatment. This is the closest we are to a safe and effective anti-ageing drug that’s perhaps only three to five years away from being on the market if the trials go well.
David Sinclair, Paul F. Glenn Center for the Biology of Aging at Harvard Medical School
Human safety trials of NMN began shortly after, and the results were published in 2020. So far, testing shows that it is safe.
Restoring youth
Shinya Yamanaka’s Nobel-prize-winning discovery of resetting adult cells to a younger state excited researchers in the anti-aging field.
But initial tests were disastrous. When Yamanaka factors were given to mice in large doses, they experienced rapid cellular growth. They developed tumors and cancer. All the mice died in days.
But in 2016, Juan Carlos Izpisúa Belmonte, professor of the Gene Expression Laboratory at the Salk Institute discovered this could be avoided. By applying a lower, and periodic dose of Yamanaka factors, cells can be restored to a younger state without being turned all the way back into stem cells and losing their identity.
That was the benefit. We don’t kill the mouse. We don’t generate tumors, but we have our rejuvenation.
Juan Carlos Izpisúa Belmonte
In 2020, a team from Stanford found they could apply a low dose of Yamanaka factors to cartilage taken from an arthritic joint. This reversed the age of the cells and alleviated the inflammation.
Members of the Stanford team formed Turn Biotechnologies, to commercialize therapies for osteoarthritis and other diseases.
Human testing
Some of these therapies require years of safety and efficacy testing before they are approved for widespread use. But there are drugs already approved for other uses that show promise in age-reversal.
In 2019, researchers created a drug cocktail of three existing drugs: lithium, trametinib, and rapamycin. Individually, each of these drugs extended the lifespan of fruit flies by an average of 11%. Taken in combination the fruit flies lived 48% longer.
In the same year, a different group of scientists reported success with a different cocktail of drugs. This time tested in humans.
The researchers combined a human growth hormone rHGH, the steroid DHEA and the diabetes medication metformin.
The researchers gave this drug cocktail to human test subjects over one year, while periodically measuring their biological clocks. In the first 9 months, participants aged at a rate of -1.6 years/year. This rate accelerated to -6.5 years/year in the final 3 months of the test.
By the year’s end, participants were 1.5 years younger than when they started the experiment. They had lost 2.5 years of age!
I’d expected to see slowing down of the clock, but not a reversal. That felt kind of futuristic.
The geneticist Steve Horvath who conducted the experiment
We might consider this drug cocktail a ‘Mark I‘ elixir of life. Future medicines and technologies can only improve from here.
Future Strategies
The seven sins of aging are known. Already we have strategies for how these seven different types of damage might be repaired.
When every form of age-related damage can be repaired, even centenarians could be restored to a youthful body of a 25-year-old.
Near-term repair strategies
Many of these strategies have already been done in mice. It is only a matter of time before we can replicate them in humans.
| Damage Type | Potential Treatments |
|---|---|
| 1. Junk inside cells | calorie restriction, enzymes, gene therapy, lasers |
| 2. Junk outside cells | phagocytosis, immunotherapy |
| 3. Too few cells | exercise, growth factors, stem cells |
| 4. Too many cells | fasting, immunotherapy, suicide genes, Sirtuin 1 |
| 5. Mutations in nDNA | telomerase, gene therapy, NAD+ |
| 6. Mutations in mDNA | backups in the nucleus |
| 7. Protein crosslinks | alagebrium |
Nanomedicine
Nanomedicine is medicine crossed with nanotechnology.
Trying to repair our bodies with blunt instruments like scalpels is like trying to repair a delicate computer chip using a screwdriver. Only so much can be done with imprecise tools.
But our ability to manipulate matter on the micro and nano scale may be key to future medical breakthroughs. We can already build computer chips with individual parts only tens of atoms across.
Swallowing the surgeon
A friend of mine (Albert R. Hibbs) suggests a very interesting possibility for relatively small machines. He says that, although it is a very wild idea, it would be interesting in surgery if you could swallow the surgeon.
Richard Feynman in “Plenty of Room at the Bottom” (1959)
You put the mechanical surgeon inside the blood vessel and it goes into the heart and “looks” around. (Of course the information has to be fed out.) It finds out which valve is the faulty one and takes a little knife and slices it out. Other small machines might be permanently incorporated in the body to assist some inadequately functioning organ.
Should we ever gain proficiency at manipulating matter at the nano-scale, then there is no damage to our bodies that we could not repair. (See: “How good can technology get?” and “Can technology make us infinitely wealthy?” for more on the promise of nanotechnology.)
Digital Immortality
Even if aging is cured, people will still die from accidents and injury. People could still be killed and lost forever.
The Insurance Information Institute estimated that in the U.S. in 2018, the annual chance of a person dying from an injury was 1 in 1,334.
| Living next N years | Probability of survival |
|---|---|
| N = 1 | 99.925% — or — 1 in 1.001 |
| N = 10 | 99.253% — or — 1 in 1.008 |
| N = 100 | 92.778% — or — 1 in 1.078 |
| N = 1,000 | 47.254% — or — 1 in 2.116 |
| N = 10,000 | 0.0555% — or — 1 in 1,801 |
As we all know from experience, when we don’t backup important files, we are asking to lose them.
But what about backing up our brains?
As long as a digital backup of your brain exists, you could survive any accident. Nanotechnology could put you back together even after the complete destruction of your old body and brain.
Death is data loss
The age-old quest for immortality is then reduced to the relatively straight-forward problem of long-term data preservation.
Already, we possess the technology to automatically scan and digitize brains. It was pioneered in 2006 by the professor of molecular and cellular biology, Jeff Lichtman, who leads researchers at Harvard’s Center for Brain Science, and his student Kenneth Hayworth, who later founded the Brain Preservation Foundation.
The technology has since been commercialized. In 2020, a collaboration of researchers from Janelia and Google’s Connectomics Research Group used it to digitize the brain of a fruit fly. It can be downloaded from the website: flycircuit.tw and fruitflybrain.org.
In this work we have achieved a dream of anatomists that is more than a century old. For at least the central brain of at least one animal with a complex brain and sophisticated behavior, we have a complete census of all the neurons and all the cell types that compose the brain, a definitive atlas of the regions in which they reside, and a graph representing how they are connected.
From “A Connectome of the Adult Drosophila Central Brain” (2020)
The circuitry of the fruit fly brain takes up only 26 MB, about 3.5% of a CD. But it’s estimated that an equivalent digitization of the circuitry of the human brain would require 20 PB, nearly a billion times more storage — or 1,000 of today’s largest hard drives.
At today’s prices, it would cost over $300,000 just for the hard drives. But the cost of storing bits drops by a factor of 1,000 every 15 years.
If the trend holds, by 2035 you could backup your brain for $300.
Longevity escape velocity
In 1900, life expectancy in the United States was 47 years. By 2000 it had risen to 75 years — an increase of 28 years over a century.
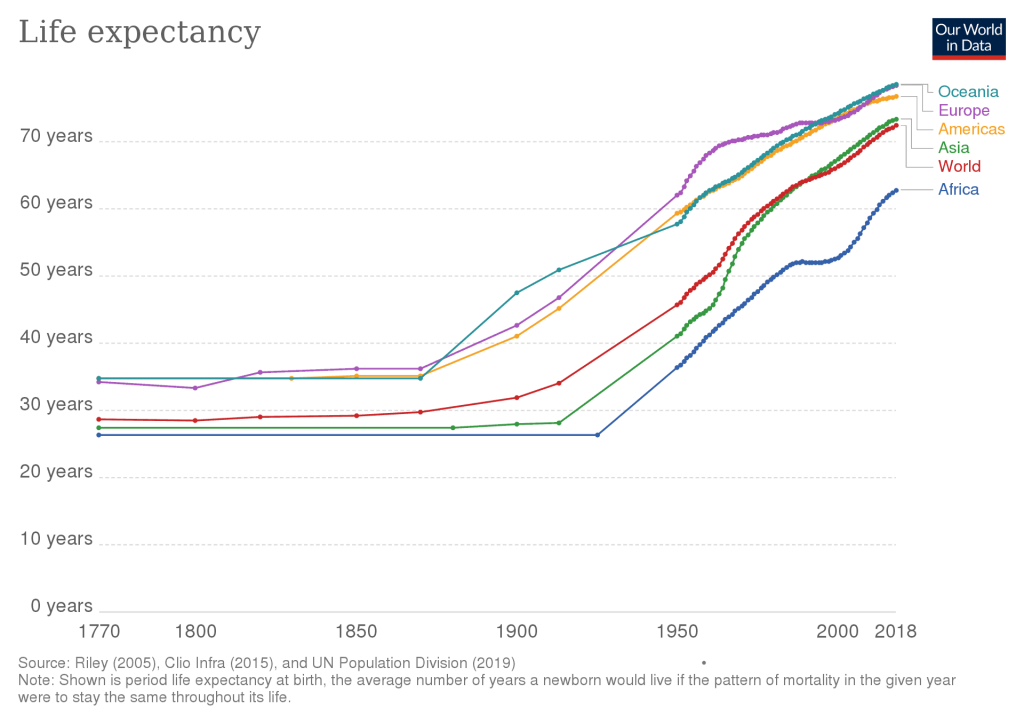
Put another way, for each year during the 20th century, life expectancy increased by 3 months.
Should future technology enable life expectancy to grow by 12 months per year, then humanity will achieve technological immortality.
Living long enough to live forever
An analysis of the history of technology shows that technological change is exponential, contrary to the common-sense ‘intuitive linear’ view. So we won’t experience 100 years of progress in the 21st century — it will be more like 20,000 years of progress (at today’s rate).
Ray Kurzweil in The Law of Accelerating Returns (2000)
Aubrey de Gray has coined this concept: longevity escape velocity.
We don’t possess technological immortality today. However, you might be young enough to live to that future where we do. Along the way we might see age reversal therapies that grant us enough extra time to make it to the point of technological immortality.
I think the first person to live to 1,000 might be 60 already.
Aubrey de Grey in 2004
How old were you in 2004? If you were under 60, then according to de Grey you could live long enough to celebrate your 1,000th birthday.
Conclusions
Every culture has a name for it: amrita, soma, ambrosia, nectar of the gods, tree of life, elixir of life, philosopher’s stone, fountain of youth.

All represent the same dream: escaping the fate of old-age and death.
Modern medicine is on the verge of finding a real fountain of youth.
We’ve extended lifespans of organisms ten-fold, used drugs to shave years off people’s age, reprogrammed cells to become youthful, and we are developing tools that could one day enable digital immortality.
Can we do it?
In 2005, MIT challenged molecular biologists to find a flaw in Aubrey de Gray’s Strategies for Engineered Negligible Senescence (SENS). They offered a $20,000 prize to whomever could make the best argument.
Despite that, it was the opinion of the judges that “no submission met the criterion of the challenge and disproved SENS.”
Feynman’s quote is as true today as it was when he spoke it: “There is nothing in biology yet found that indicates the inevitability of death.”
This is confirmed by the discovery of immortal species — they show it is possible to repair all the damage that comes with age. We know it can be done. It’s then only a matter of time until we figure it out.
Should we do it?
The less certain question is not whether we can do it, but should we?
Some say to use such powers is unnatural, that it is playing god, or that it will lead to disastrous overpopulation and resource depletion.
Life extension is unnatural
By this measure, every technology — from books, to air conditioning, to soap — is unnatural. But it is our nature to transcend our natural limits.
Why stop with aging, when it causes so much suffering?
We will transcend death and that natural cycle. We’re not just grapes on the vine—we are overcoming that natural process that we emerged from. Yes, we came from nature, but we are going to surpass it through the power of our technology, which comes from our mind made manifest in the real world.
Ray Kurzweil
Overpopulation
The Roman philosopher Lucretius argued two-thousand years ago that death was good, because it made room for the next generation.
Up, with good grace! make room for sons: thou must.
Titus Lucretius Carus in “On the Nature of Things” (circa 60 B.C.)
Justly, I fancy, would she reason thus,
Justly inveigh and gird: since ever the old
Outcrowded by the new gives way, and ever
The one thing from the others is repaired.
But this reasoning, that curing death inevitably leads to overpopulation, overcrowding, resource depletion and environmental destruction ignores possibilities that technology makes possible.
Consider the following example from history:
Suppose you’re a scientist 200 years ago who has figured out how to drastically lower infant mortality with better hygiene. You give a talk on this, and someone stands up in back and says, “Hang on, if we do that we’re going to have a population explosion!” If you reply, “No, everything will be fine because we’ll all wear these absurd rubber things when we have sex,” nobody would have taken you seriously. Yet that’s just what happened–barrier contraception was widely adopted” about the time that infant mortality began dropping.
Aubrey de Grey in Fortune (2004)
Technological solutions
The technologies that enable nanomedicine and digital immortality are the very same tools that can solve problems of overpopulation, overcrowding, and resource depletion — all while allowing the world’s population of humans to increase by a factor of millions.
Our current approach to growing food is enormously inefficient. It takes an acre of farmland to feed each person. Over the day, this acre of farmland receives an average of 663,684 watts of solar energy.
If we used this energy to directly synthesize food, e.g. with nanotechnology, we could feed 6,853 people!
Food synthesis technology will enable us to drastically reduce our footprint on the environment, while at the same time support much greater human populations.
What about overcrowding?
Humanity, as it turns out, does not take up much space. Every human on earth, all 7.8 billion of us, could fit in a building one cubic mile in volume and 1,000 of these buildings would fit in the grand canyon.
The reason we face overpopulation and resource depletion today is that our food production is so space and energy inefficient.
(See: “What are the limits of human population growth?“)
Future technologies, like mind uploading will not only provide each person unlimited space in virtual reality but also enable people to live anywhere. For instance: on the moon.

The moon receives 13,000 Terawatts of solar energy. Since the human brains uses 20 watts of power, this is enough energy to power 650 trillion human souls — 83 thousand times Earth’s current population.
We could leave Earth and allow the environment to heal.
Perhaps this has happened already. (See: “Are we living in a computer simulation?“)
Will we do it?
There are compelling reasons to try. The upside is huge.
People working on it
Many organizations are working on, if not devoted to, a cure for aging.
This includes the SENS research foundation, Google-backed Calico, Human Longevity, Harvard’s Paul F. Glenn Center for Biology of Aging Research, UCLA’s Molecular Biology Institute, the American Academy of Anti-Aging Medicine, BioViva, Turn Biotechnologies, Unity Biotechnology, The Methuselah Foundation, and Age X, to name a few.
Must we do it?
Perhaps the question isn’t can we? or should we?, but mustn’t we?
Every day 100,000 people die from age-related illnesses. Do we not have a moral imperative to save these people?
We will do it!
What old person doesn’t wish to be younger? Which sick person doesn’t want to be healthy? When has humanity ever not tried something that was possible?
There is overwhelming demand for restoring youth and extending lifespan. Perhaps not everyone will go for it, but some will. The option to spend longer than 120 years on this planet will become available.
The implication is that if you can live long enough, you will be able to live forever — or at least — as long as you want.
But is it necessary to try to prolong life in the here and now of this universe? That depends. (See: “Is there life after death?“)




Thank you, this was a great read.
Thank you Valen! I am glad you enjoyed it.
Thak you for your pen. Great job. l found some answers for questions without answers. But still have many questions becouse it is said that semi part of theorical physic is lie and other part of physic is
bragging. Thank you so much!
Hi Semih,
Thank you! If you have other questions that you want answered and think would make good topics for the site, I welcome you to add suggestions on the questions page:
https://alwaysasking.com/questions/
Best wishes,
Jason